Understanding CT Pixel Values in Medical Imaging: A Practical Guide to Hounsfield Units (HU)
Computed Tomography (CT) is one of the most widely used imaging modalities in clinical radiology, emergency medicine, and AI-driven diagnostics. Unlike natural images, CT scans encode quantitative tissue density using Hounsfield Units (HU)—a standardized scale critical for both clinical interpretation and medical AI model performance.
For radiologists, HU enables accurate differentiation between tissue types and pathologies.
For AI teams, HU consistency is essential for robust training, multi-center generalization, and regulatory-grade reliability.
This article explains how CT pixel values work, why HU matters in medical imaging, and how HU-aware workflows enable production-ready AI systems.
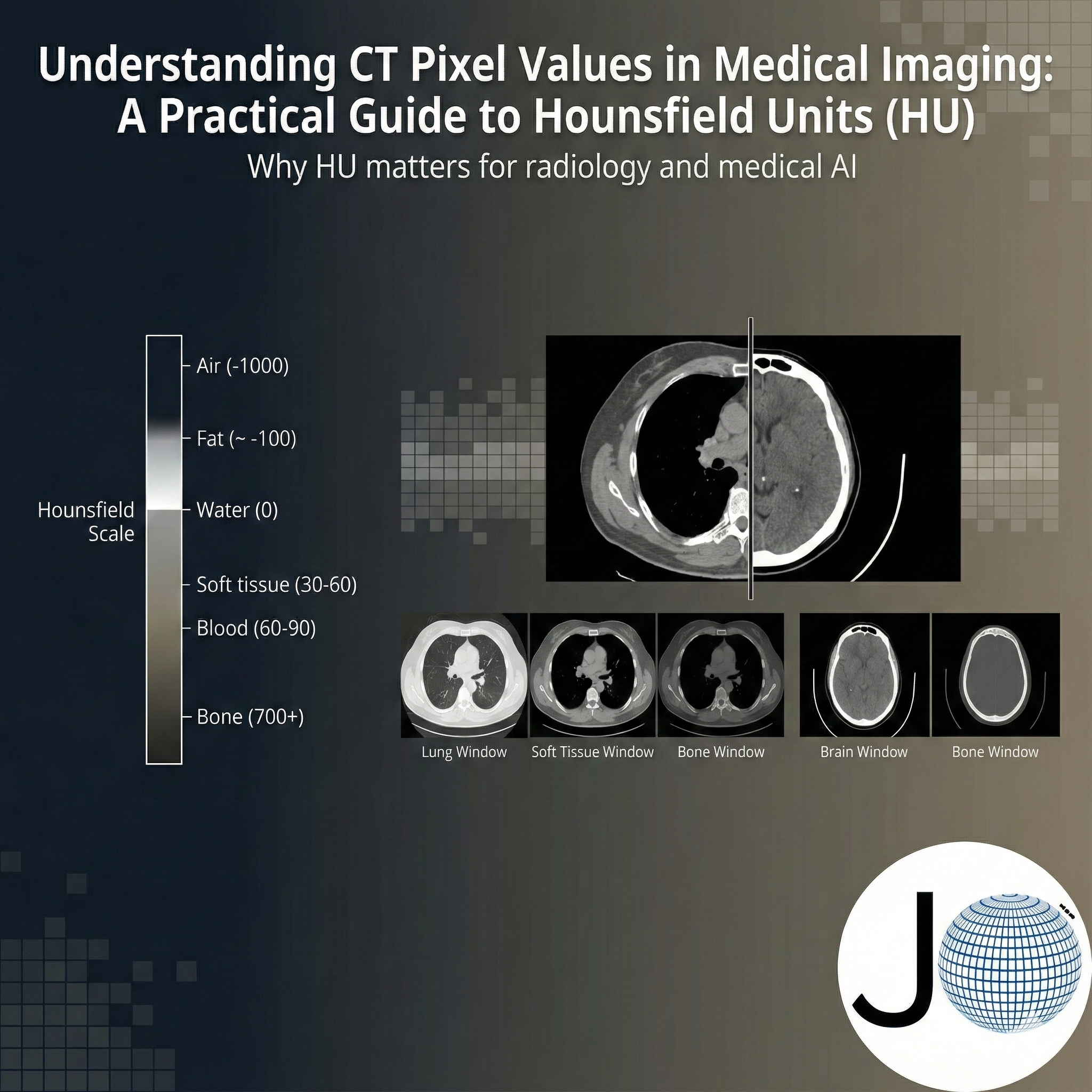
What Are Hounsfield Units in CT Imaging?
Hounsfield Units (HU) represent the X-ray attenuation properties of tissues relative to water and air.
The HU scale is globally standardized:
- Air: −1000 HU
- Water: 0 HU
- Dense cortical bone: +1000 HU and above
This calibration ensures that a given tissue appears at comparable HU values across scanners, within expected clinical variance.
This is why CT is considered a quantitative imaging modality, not merely a visual one.
Why HU Is Critical in Clinical CT Interpretation ?
Raw CT detector values are influenced by:
- Scanner manufacturer and hardware
- Acquisition parameters (kVp, reconstruction kernel)
- Patient-specific factors
Without HU normalization:
- Soft tissue contrast becomes unreliable
- Density-based diagnosis is inconsistent
- Cross-institution comparison is impossible
Hounsfield Units solve this by enabling:
- Reliable tissue differentiation
- Density-based diagnosis (e.g., hemorrhage vs ischemia)
- Objective measurements for follow-up studies
Clinically Relevant HU Ranges in CT
| Tissue / Pathology | Typical HU Range | Clinical Relevance |
|---|---|---|
| Air | −1000 | Lung fields |
| Lung Parenchyma | −700 to −500 | Emphysema, consolidation |
| Fat | −120 to −90 | Fat planes, lipomas |
| Water | 0 | Reference baseline |
| CSF | 0 to +15 | Hydrocephalus |
| Soft tissue / Muscle | +20 to +60 | Organs, muscle |
| Acute hemorrhage | +60 to +80 | Stroke, trauma |
| Cancellous bone | +200 to +400 | Vertebrae |
| Cortical bone | +700 to +2000 | Fractures |
| Metal / Contrast | > +3000 | Implants, contrast agents |
Approximate HU ranges (non-contrast CT).
Note: Acute hemorrhage HU values listed above assume non-contrast CT. In contrast-enhanced scans, iodine-based contrast agents increase attenuation, shifting HU values for blood, vessels, and soft tissues.
How CT Hounsfield Units Are Computed
HU values are calculated using linear attenuation coefficients:
HU = 1000 × (μ_tissue − μ_water) / (μ_water − μ_air)
This formulation guarantees:
- Water is always 0 HU
- Air is always −1000 HU
This mathematical grounding is what makes CT suitable for quantitative diagnostics and AI analysis.
CT Windowing: Visualization vs Quantification
CT images contain a wider dynamic range than standard monitors can display. Windowing maps a selected HU range to grayscale for human interpretation.
Core Parameters
- Window Level (WL): Center HU
- Window Width (WW): HU range
Common Radiology Windows
| Clinical Use | WL | WW |
|---|---|---|
| Brain | 40 | 80 |
| Lung | -600 | 1500 |
| Soft Tissue | 40 | 400 |
| Bone | 500 | 2000 |
⚠️ Windowing affects visual appearance only.
The underlying HU values remain unchanged—critical for AI and measurements.
HU in DICOM CT Files: Developer & AI Perspective
In DICOM CT images:
- Pixel data is stored as raw integer values
- HU conversion requires:
- RescaleSlope
- RescaleIntercept
Mandatory Conversion Formula
HU=(PixelValue×RescaleSlope)+RescaleInterceptHU = (PixelValue \times RescaleSlope) + RescaleInterceptHU=(PixelValue×RescaleSlope)+RescaleIntercept
This step is essential before:
- Training medical AI models
- Performing HU-based segmentation
- Applying diagnostic thresholds
Failure to apply this conversion leads to invalid models and misleading results.
Why HU Consistency Is Essential for Medical AI
CT-based AI models rely on HU to learn true tissue characteristics, not scanner artifacts.
Benefits for AI Systems
- Scanner-agnostic training
- Stable feature distributions
- Improved generalization across hospitals
- Clinically interpretable predictions
High-Impact AI Use Cases
- Intracranial hemorrhage detection
- Lung nodule analysis
- Organ segmentation (liver, kidneys, lungs)
- Trauma assessment
- Bone density evaluation
Standard HU Preprocessing in Medical AI Pipelines
Production-grade pipelines typically include:
- Raw pixel → HU conversion
- HU clipping (e.g., −1000 to +1000)
- Normalization to model-friendly ranges
- Task-specific windowing (optional, visualization only)
Improper HU handling is among the top causes of AI model failure in radiology.
Common Pitfalls in CT AI Workflows
- Skipping RescaleSlope / RescaleIntercept
- Training on windowed PNG/JPEG images
- Mixing multi-scanner data without HU normalization
- Using hard-coded HU thresholds without validation
How JTheta.ai Supports HU-Accurate CT Workflows
JTheta.ai is designed specifically for medical imaging and clinical AI teams:
- Native DICOM CT ingestion
- Automatic HU conversion at load time
- Radiology-grade window presets
- Preservation of raw pixel integrity
- HU-consistent datasets for annotation, training, and validation
This ensures that annotations and AI models are built on clinically valid, quantitative data.
Final Takeaway for Medical Imaging Teams
Hounsfield Units are the backbone of CT imaging.
Without HU-aware workflows, both clinical interpretation and AI outcomes are compromised.
As medical AI moves toward regulatory approval and real-world deployment, HU consistency is no longer optional—it is foundational.
Learn More with JTheta.ai
Explore expert insights on:
- Medical imaging AI
- CT, MRI, and DICOM workflows
- AI-assisted annotation for radiology
- Clinical-grade visualization standards
Build reliable, HU-accurate CT AI workflows with JTheta.ai.